Hemoglobinís Oxygen Carrying Capacity
Magnesium serves hundreds of important
functions in the body and one of them has to do with the efficiency
of red blood cells and their capacity to carry oxygen. Researchers have
investigated the effect of dietary magnesium (Mg) deficiency on the
nutritive utilization and tissue distribution of iron (Fe). Magnesium
deficient diet leads to significant decreases in the concentration of
red blood cells (RBC), hemoglobin and eventually a decrease in whole
blood Fe. In fact we find many ways in which magnesium deficiency leads
to problems with oxygen transport and utilization.1
Chronic Mg deficiency has also been
shown to increase copper absorption and concentrations in plasma, muscle,
kidney, and liver.2 Magnesium is involved
with the transport gases across the red blood
cell membranes as well as ions, amino acids, nucleosides, sugars,
and water. Magnesium levels drop more slowly in red blood cells than
in the serum.3
A study by physiologist Henry C. Lukaski
and nutritionist Forrest H. Nielsen (Grand Forks Human Nutrition Research
Center) reveals important findings on the effects of depleted body magnesium
levels on energy metabolism. The data shows that magnesium
deficient people used more oxygen during physical activity -their
heart rates increased by about 10 beats per minute. “When the
volunteers were low in magnesium, they needed more energy and more
oxygen to do low-level activities than when they were in adequate-magnesium
status,” says Lukaski.4
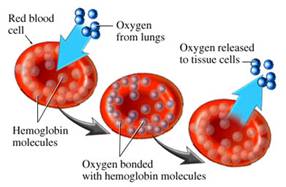
“The transport of oxygen
in blood is undertaken by hemoglobin, the largest component of red blood
cells. This protein collects oxygen in respiratory organs, mainly in
the lungs, and releases it in tissues in order to generate the energy
necessary for cell survival. Hemoglobin is one of the most refined proteins
because its evolution and small mutations in its structure can produce
anemia and other severe pathologies,” publishes the Institute
for Research in Biomedicine (IRB Barcelona).
They continue, “More than a hundred
years of study have led to the knowledge that hemoglobin uses mechanisms
of cooperatively to optimize its function; that is to say, to collect
the greatest amount of oxygen possible in the lungs and release it in
tissues. These mechanisms of cooperatively are related to changes in
the structure of the hemoglobin protein.”
The structure
of hemoglobin is easily compromised by heavy metals like mercury
(as are all sulfur bearing proteins 5
like insulin etc). In my book NewParadigms in Diabetic Medicine we nail
down the mercury sulfur bond death destruction scenario. You can bet
your last medical dollar on the fact that high magnesium and selenium
status is protective of red blood cells and thus of total oxygen carrying
capacity.
The mechanism whereby red cells maintain
their biconcave shape has been a subject of numerous studies. One of
the critical factors for the maintenance of biconcave shape is the level
of red cell adenosine triphosphate (ATP) levels.The interaction of calcium,
magnesium and ATP with membrane structural proteins exerts a significant
role in the control of shape of human red blood cells.6
Magnesium enhances the binding of oxygen to haem
proteins.7 The concentration
of Mg2+ in red cells is relatively high but free Mg2+ is much lower
in oxygenated red blood cells then in deoxygenated ones. This suggests
some kind of magnesium pump where oxygen climbs aboard the red cells
and magnesium jumps off only to have to jump right back on again.
Dr. L.O. Simpson asserts that Chronic
Fatigue Immune Deficiency Syndrome (CFIDS), results from "insufficient
oxygen availability due to impaired capillary blood flow." This
would naturally reflect to the mitochondria who would be having their
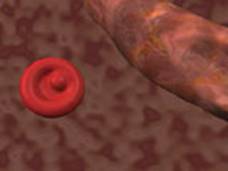
O2 deprivation problems. In healthy people, most red blood cells are
smooth-surfaced and concave-shaped with a donut-like appearance. These
discocytes have extra membranes in the concave area that give them the
flexibility needed to move through capillary beds, delivering oxygen,
nutrients, and chemical messengers to tissue and removing metabolic
waste, such as carbon dioxide and lactic acid.
Red blood cells are also known as
erythrocytes. They have a unique shape
known as a biconcave disk. A biconcave disk is like a donut where the
hole
doesn’t go all the way through. The biconcave
disk shape increases the
surface area of the cell which allows for a greater area for gas exchange.
Abnormal magnesium
deprived red blood cells lack flexibility that allow them to enter tiny
capillaries. These nondiscocytes are characterized by a variety
of irregularities, including surface bumps or ridges, a cup or basin
shape, and altered margins instead of the round shape found in discocytes.
When people become ill or physically stressed (more magnesium deficient),
a higher percentage of discocytes transform into the less flexible nondiscocytes.
Magnesium stimulates the movement
of
oxygen atoms from the bloodstream to the cells.
Magnesium and zinc prevent the binding
of carbon monoxide/CO to haem which otherwise binds 25,000 times more
strongly than does oxygen. The dissociation of oxygen is also helped
by magnesium, because it provides an oxygen adsorption isotherm which
is hyperbolic. It also ensures that the oxygen dissociation curves are
sigmoidal which maximizes oxygen saturation with the gaseous pressure
of oxygen (Murray et al pp. 65-67).
Oxygen dissociation with increased delivery
to the tissues is increased by magnesium through elevation of 2,3-bisphosphoglycerate/DPG
(Darley, 1979) Magnesium stabilizes the ability of the phorphyrin ring
to fluoresce. Free-radical attack of haemoglobin yields ferryl haemoglobin
[HbFe4+] (D’Agnillo and Alayash, 2001), which is inhibited by
magnesium (Rock et al, 1995).
Magnesium prevents blood vessels from
constricting, thus warding off
rises in blood pressure, strokes and heart attacks. Magnesium inhibits
the
release of thromboxane, a substance that makes blood platelets stickier.
Dr. Jerry L. Nadler
Low red blood cell magnesium levels,
a more accurate measure of magnesium status than routine blood analysis,
have been found in many patients with chronic fatigue. Red blood cell
(RBC) deformability is an important factor in determining movement of
red blood cells through the microcirculation. Intravenous magnesium
therapy over a 24-hour period has been shown to increase RBC-deformability
even in pregnancies with normal RBC-deformability. An increase of RBC-deformability
with magnesium administration offers therapeutic benefit for the treatment
of reduced blood flow seen in most cases of preeclampsia.8
D F Treacher and R M Leach teach, “Oxygen
transport from environmental air to the mitochondria of individual cells
occurs as a series of steps. The system must be energy efficient (avoiding
unnecessary cardiorespiratory work), allowing efficient oxygen transport
across the extravascular tissue matrix. At the tissue level, cells must
extract oxygen from the extracellular environment and use it efficiently
in cellular metabolic processes.”
Patients with chronic fatigue syndrome
(CFS) have low red blood cell magnesium. The physiological concept of
fatigue as a consequence of inadequate oxygen delivery is accepted tying
oxygen carrying capacity directly to magnesium. This is good medicine
to understand and appreciate.
Magnesium-deficiency studies on the
kidneys have shown intraluminal calcareous deposits in the corticomedullary
area and damage to the tubular epithelium. Damage to the kidneys from
magnesium deficiency creates a situation that intensifies magnesium
deficits. Micropuncture studies have shown that most active renal tubular
reabsorption of magnesium occurs at sites that are potentially damaged
by magnesium deficiencies meaning these conditions can cause renal tubular
magnesium wasting. Both hyper-parathyroidism and hypervitaminosis D
increase blood and thus urinary loads of calcium and thus cause even
further magnesium loss.
Most renal reabsorption of magnesium
occurs in the proximal tubule and the thick ascending limb of the loop
of Henle. In hypomagnesemic patients, the kidney may excrete as little
as 1 mEq/L of magnesium. Magnesium will be removed from bone stores
in times of deficiency. Primary renal disorders cause hypomagnesemia
by decreased tubular reabsorption of magnesium by the damaged kidneys.
This condition occurs in the diuretic phase of acute tubular necrosis,
postobstructive diuresis, and renal tubular acidosis.
Any study of human physiology will yield
up magnesium’s vital role in keeping us healthy. Magnesium chloride
is the heavy weight champion of the pharmaceutical world. Magnesium
chloride sounds like something alien, forbidden or too medical like
to those unfamiliar with its medicinal charm (it’s the beautiful
metal in Chinese). In each and every medical situation we find that
magnesium chloride (common magnesium form from sea water) is the necessary
foundational medicine.
Magnesium supports all further intervention
into the human bio-system through whatever means. From facilitating
massage (combining medical massage with transdermal magnesium therapy)
to enhancing surgery, from the emergency room to the intensive care
ward, for treating ones water to strengthening one against the flu,
it’s a potent medicine that can be used in many ways. It should
almost always be used with pharmaceutical drugs because many of them
further waste magnesium.9 When we waste
magnesium through the kidneys we are wasting away our very life force.
Our metabolism declines; oxygen carrying capacity is decreased, cell
respiration suffers, insulin action and cell receptivity to insulin
goes into decline and we age that much faster.
When life’s struggles get
more intense we need friends to support and strengthen us. Magnesium
Oil (magnesium chloride) is such a friend and we should be stocking
up on that along with some other precious medicinals like iodine
and sodium
bicarbonate in our medicine chests. These are the holy medical trinity
in my medical approach called Natural
Allopathic Medicine and there are many book long dissertations of
mine to show why these three are the most important Survival
Medicines for the Twenty First Century.
Mark Sircus Ac., OMD
Director International Medical Veritas Association
http://publications.imva.info
Click on the books for information.

_________________________________________
1 Influence of magnesium deficiency on
the bioavailability and tissue distribution of iron in the rat. The
Journal of Nutritional Biochemistry, Volume 11, Issue 2, Pages 103-108
2 J. Agric. Food Chem., 1997, 45 (10),
pp 4023–4027 DOI: 10.1021/jf970011k
pubs.acs.org/doi/abs/10.1021/jf970011k
3 www.jbc.org/cgi/reprint/122/3/693.pdf
4 www.agclassroom.org/teen/ars_pdf/family/2004/05lack_energy.pdf
5 It has long been known that the sulfur
contents of hemoglobins of different species vary. Therefore one or
both of the sulfur containing amino acids must exist in different quantities
in the various globins.
6 bloodjournal.hematologylibrary.org/cgi/reprint/44/4/583.pdf
7 Terwilliger and Brown, 1993; Takenhiko
and Weber; Wood and Dalgleish, 1973
8 www.informahealthcare.com/doi/abs/10.1081/PRG-45767?cookieSet=1&journalCode=hip
9 These include oral contraceptives
and both conjugated estrogens (Premarin) and esterified estrogens (Estratab),
various antibiotics, such as tetracyclines and doxycline. Diuretics
are another class of magnesium-depleting drugs, as is digoxin, used
in the treatment of congestive heart failure. Corticosteroids also deplete
magnesium as do certain cisplatin chemotherapy drugs.
Legal Notice:The Author specifically invokes
the First Amendment rights of freedom of speech and of the press without
prejudice. The information written is published for informational purposes
only under the rights guaranteed by the First Amendment of the Constitution
for the United States of America, and should not in any way be used
as a substitute for the advice of a physician or other licensed health
care practitioner. The statements contained herein have not been evaluated
by the FDA. The products discussed herein are not intended to diagnose,
cure, prevent or treat any disease. Images, text and logic are copyright
protected. ALL rights are explicitly reserved without prejudice, and
no part of this essay may be reproduced except by written consent. ©2009
by Mark Sircus
|